Digital PCR Development for Early Detection of Cancer and Dementia, RevoSketch Co., Ltd.
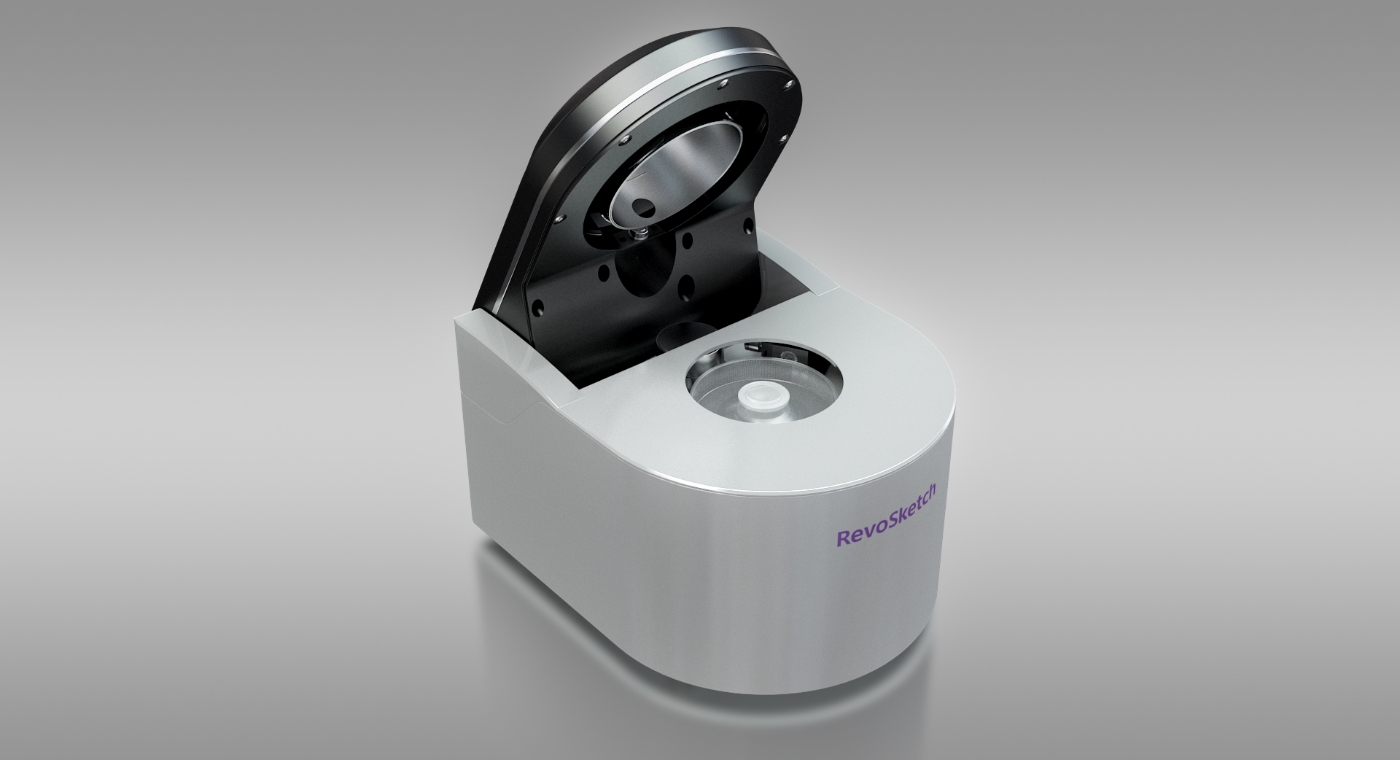
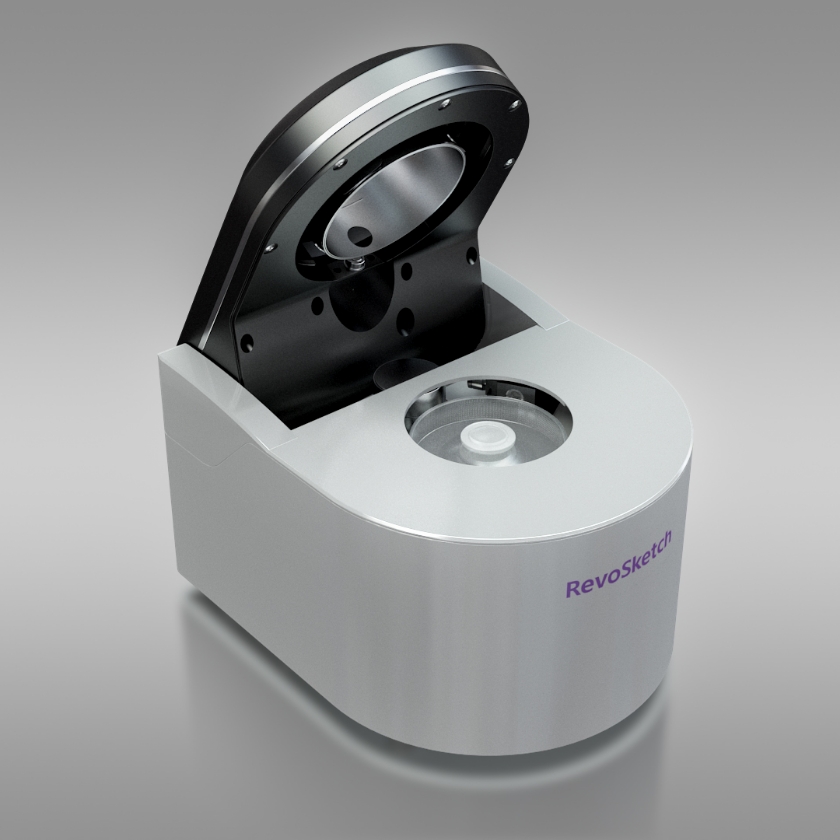
RevoSketch Co., Ltd. is committed to extending humanity's healthy lifespan by developing next-generation medical devices. By leveraging over 20 years of expertise in laser and fluorescence detection technologies, we tackle challenges previously deemed insurmountable with existing technologies. Over the past century, medical advancements have been staggering, resolving numerous diseases and propelling the world into an era of an aging population. The human lifespan peaks at around 120 years, but we still grapple with threats from incurable age-related diseases such as cancer and Alzheimer's. In response, RevoSketch strives to significantly contribute to the cure of these refractory diseases, ensuring that in the near future, South Korea will play a pivotal role in the global medical market. Our unyielding commitment is to persist with research and development until humanity surpasses a lifespan of 500 years, reaching a state of everlasting life.
Business Areas
Digital PCR
This is the most advanced third-generation PCR technology. Compared to the widely-used second-generation Real-time PCR, it boasts over 1000 times higher sensitivity, allowing for nucleic acid quantification.
Its enhanced sensitivity is anticipated to be vital in detecting minimal genetic variations, which were difficult to discern with second-generation Real-time PCR, paving the way for early diagnosis in cancer and dementia (Alzheimer's) sectors.
Isothermal PCR (LAMP PCR)
Based on isothermal amplification technology, the isoQuarkF2 and isoQuarkM4 diagnose diseases by maintaining samples at specific temperatures to amplify cells or infectious agents' nucleic acids (DNA or RNA). The fluorescence detector confirms real-time amplification and detects fluorescent substances bound to genes.
Immune Diagnosis
This device simplifies the complex ELISA technique, making testing more accessible and cost-effective. It can detect and quantify bio-markers based on protein for diagnosing infectious diseases and breast cancer.




 Wellness Tour
Wellness Tour
 Bio Technology
Bio Technology
 Medical tour tip
Medical tour tip
 Festival & Mice
Festival & Mice
 Hot Issue
Hot Issue
 Interview With
Interview With
 Medical Technology
Medical Technology
 City & Culture
City & Culture
 Food & Travel
Food & Travel
 Health & Wellness Tips
Health & Wellness Tips
 Hot Issue
Hot Issue