Genetic Analysis for Disease Diagnosis and Treatment of Intractable Diseases
The healthcare industry, which has been reliant on medical professionals and their expertise, is turning into a tech-based industry that uses big data of genetic information for diagnosis and treatment. This is especially evident in the areas of molecular diagnostics and RNAi therapy. These days, it is possible to diagnose, treat and even prevent diseases by analyzing the DNA, which contains genetic information. This is quite a change from the days when doctors examined patients using just a stethoscope and palpation, and it signals the arrival of an era of personalized medical care based on genetic analysis.
Bioneer Corp. is the one and only provider of a “total solution for molecular diagnostics” in Korea. The company has independently developed the equipment, kits and reagents for molecular diagnostic tests using around 200 patented technologies. They have secured a competitive advantage in terms of pricing and distribution, with the capability to manufacture everything they need, from raw materials to state-of-the-art equipment, on their own. Based on a turnkey lab operation, they are exporting to more than 90 countries around the world.
Using the molecular diagnostic kits from Bioneer Corp., it is possible to obtain accurate results using trace amounts of samples. The company has developed diagnostic kits for a wide range of applications, including COVID-19 testing as well as AIDS, hepatitis, tuberculosis and STD testing, organ transplantation screening, cancer screening and hereditary disease screening. They currently have more than 40 different molecular diagnostic kits in their product line.
ExiStation™ is an automated molecular diagnostic system from Bioneer Corp. for extraction, amplification and analysis of nucleic acids from samples. It boasts excellent performance and convenience, and it is also compact in size. With a product lineup of diverse nucleic extraction equipment and real-time PCR equipment, Bioneer is capable of supplying an optimized system for each customer according to their needs in relation to test volume and types.
The molecular diagnostic kits developed by Bioneer Corp. are of world-class standards. The COVID-19 diagnostic kit, in particular, has been evaluated to exhibit 100% sensitivity by the Foundation for Innovative New Diagnostics (FIND). The company also became the world’s first to receive the Emergency Use Authorization (EUA) by the World Health Organization (WHO) for a multi-diagnostic kit for the Zika virus, and it is the sole manufacturer in Asia to obtain the CE marking for in vitro diagnostic medical devices (IVDs) for AIDS, hepatitis B and hepatitis C (List A).
*FIND: A non-profit international organization that evaluates diagnostic products by carrying out in-house clinical experiments. They are known for the transparency and reliability of their evaluation results, attested by the fact that the health departments of countries around the world as well as WHO and the US Centers for Disease Control and Prevention refer to their reports when purchasing diagnostic products.

Bioneer plans to launch IRON-qPCR™, a point-of-care (POC) molecular diagnostic platform suitable for small- and mid-sized clinics and laboratories performing emergency testing, and ExiStation™ 96 FA, an automated molecular diagnostic system that has been optimized for use at hospitals and large testing centers. These products are expected to provide solutions for customers with different needs.
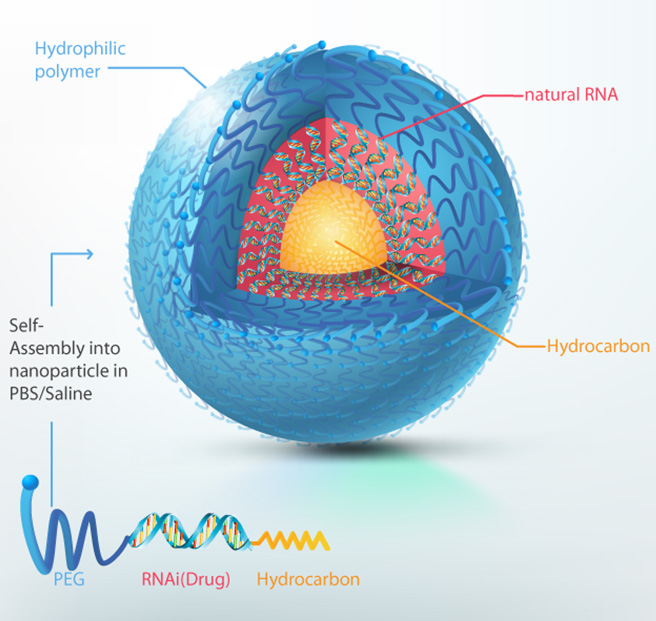
Genetic analysis is also performed in the development of new drugs for treatment of intractable diseases. RNA interference (RNAi) therapy involves selectively targeting genes to inhibit the production of disease-causing proteins. Unlike conventional medications, RNAi agents have the potential to treat intractable diseases. However, in the past, there have been a number of obstacles to developing RNAi agents for this purpose, as they must be delivered to the target organ or tissue without undergoing degradation in the body and exhibit no adverse effects such as toxicity.
In order to overcome these limitations, Bioneer has developed SAMiRNA™, an RNAi new drug platform. SAMiRNA™ is stable and reaches the target site, such as an inflamed area or tumor, without break down in the bloodstream. It has also been proven to be safe and effective in a non-clinical study and a toxicity study using primates.
Bioneer will develop a new drug candidate material, SAMiRNA-AREG, for treatment of fibrosis, with plans to begin Phase 1 clinical trial this year. When tested on animals, the effect of symptom alleviation and improvement was seen, and a paper on the study has been published in 2016 in the Journal of Biological Chemistry (JBC) and Scientific Reports, an international journal published by Nature. We hope that Bioneer’s SAMiRNA™ start being used to treat intractable diseases and cancer and help promote the health of all mankind.





 Specialized Medical Service
Specialized Medical Service
 Medical Technology
Medical Technology
 Health & Wellness
Health & Wellness
 City & Culture
City & Culture
 Hot Issue
Hot Issue
 Interview With
Interview With
 Medical Technology
Medical Technology
 City & Culture
City & Culture
 Food & Travel
Food & Travel
 Health & Wellness Tips
Health & Wellness Tips
 Hot Issue
Hot Issue